For more than 70 years, doctors treated the symptoms of schizophrenia—delusions, hallucinations, cognitive impairments—with antipsychotic medications. Prevailing theories suggest that elevated dopamine signaling in the brain leads to schizophrenia, so these antipsychotics provide relief by tempering dopamine activity. Yet, it has never been entirely clear how these drugs quiet dopamine activity. And due to their nature, these drugs impact other parts of the body and foster unwanted side effects including weight gain, constipation, and drowsiness. On top of that, more than nearly a third of patients don’t even respond to two or more common antipsychotic treatments.
What if there was a better way to treat the more than 24 million people around the world with schizophrenia? A new study run by researchers in Japan and published earlier this year in Cell Reports Medicine suggests that for at least a significant portion of patients, the immune system is mistakenly attacking a protein in the brain—which may be the real mechanism giving rise to schizophrenic symptoms in the first place.
This study is the tip of the iceberg too.
“We don’t know what causes schizophrenia,” Roger McIntyre, psychiatrist and professor of psychiatry and pharmacology at the University of Toronto (who was unaffiliated with this work), told The Daily Beast. “Rigorous scientific studies that have been conducted and they have concluded that for some people, some of the symptoms of schizophrenia may be a consequence of a disturbance in the immune inflammatory system.”
“
What’s most debilitating is the cognitive symptoms: that is the ability to focus, concentrate, your memory, your processing speed, and also what’s called negative symptoms. In other words, people who have schizophrenia often have this lack of motivation.
”
—
Roger McIntyre, University of Toronto
This in turn, may open the door to an entirely new way of treating schizophrenia—one that’s unencumbered by the challenges holding back current antipsychotics.
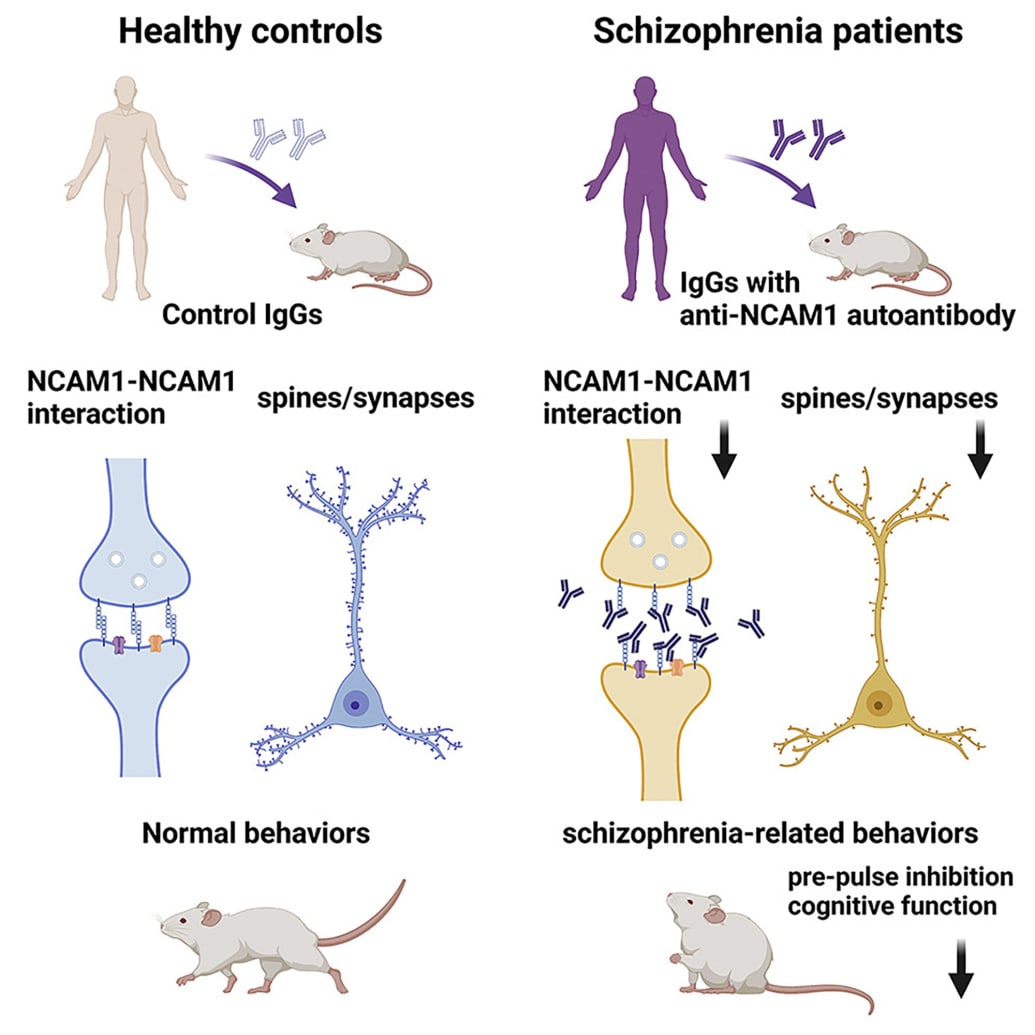
The team behind the new study analyzed blood from about 200 patients with schizophrenia and compared it to blood samples from more than 200 healthy individuals. In about 6 percent of schizophrenia patients, the researchers found elevated levels of an antibody that targeted NCAM1, a protein crucial for cell communication in the brain. None of the healthy individuals enrolled in the study produced this antibody.
Hiroki Shiwaku, et al.
That’s a surprising finding, but was this antibody actually responsible for the symptoms themselves? Or just another marker of the condition?
The researchers isolated the antibodies and injected them into mice. In addition to causing cognitive impairments and other behaviors characteristic of schizophrenia, the NCAM1 autoantibodies also reduced the number of connections in the brain.
“Even though the mice only had these autoantibodies in their brains for a short time, they had changes in their behavior and synapses that were similar to what is seen in humans with schizophrenia,” Hidehiko Takahashi, a professor of psychiatry and schizophrenia researcher at Tokyo Medical and Dental University and co-author of the new study, said in a press release.
McIntyre added that there’s other connections between schizophrenia and the immune system, including a subset of patients who have antibodies against other proteins in the brain, like the NMDA receptor or other signs of immune dysregulation. “People who live with schizophrenia or bipolar disorder or depression are at higher risk of contracting COVID-19, ending up in hospital and dying,” he said, citing an earlier study his research group published in JAMA Psychiatry.
A 2018 study found that people with an existing autoimmune disorder are more than 70 percent more likely to develop schizophrenia. This is not too surprising given that prominent genetic risk factors for the disease occur as mutations on a gene locus called the major histocompatibility complex. This complex is important for teaching the immune system the difference between the body’s own proteins and invading pathogens.
And the immune system-schizophrenia evidence goes beyond genetics. The brain’s resident immune cells, the microglia, have also been implicated in the development and pathology of the disease.
“
There are certain forms of breast cancer, you can genotype somebody, and based on the genotype, you can determine whether they are especially going to respond to certain drugs, for example, breast cancer. We’re not there yet in psychiatry, but that’s exactly the direction people want to go.
”
—
Roger McIntyre, University of Toronto
Microglia are the avid plant-enthusiasts and gardeners of the brain. As neurons sprout and grow early in life, the microglia take care to cut off any unused or unneeded connections. Then they provide continued support by dealing with pathogens and infection, clearing any debris, and getting rid of dysfunctional cells. Like the handiwork of a terrible gardener, microglia can leave behind a trail of mayhem.
Maternal infection during pregnancy is known to increase the risk of developing schizophrenia—animal models implicate microglia as the mediators of this effect. In post-mortem studies, researchers found elevated levels of activated microglia “attacking” other cells in the brain.
And that’s not all—they also release and respond to many signaling proteins called cytokines which may also play a role in schizophrenia. In 2011, researchers discovered that the levels of several cytokines are either higher or lower than the levels found in healthy individuals. Some cytokines, including interleukin-1β and interleukin-6 were elevated after a first psychotic episode as well as in patients experiencing a relapse of symptoms. Antipsychotic treatments also lowered the levels of these proteins, bolstering the idea that these cytokines are targets for new treatments.
All of that research has led to a very important question: Could existing medications that reduce inflammation or target specific cytokines boost the response to antipsychotic medication?
Results in 2014 showed that a broad approach to treat overall inflammation in patients with schizophrenia found that taking aspirin, estrogen, or an antioxidant called N-acetylcysteine in addition to antipsychotic medication had a small but meaningful impact on symptom severity. Zeroing in on microglia, other researchers discovered in 2017 that taking antipsychotics along with minocycline—a drug that prevents microglia from activating—also improved overall symptoms.
According to McIntyre, minocycline is often prescribed as an off-label treatment. “You're not going to see minocycline as a recommended treatment because this is very much outside the usual practice,” he said. “But clinicians spend a lot of their time treating patients who are not benefiting from the usual practice.”
Since individuals with schizophrenia can experience a wide range of symptoms and pathologies, scientists are working toward developing more specific, personalized treatments. “We really think that schizophrenia is a group of disorders there’s different biological causative pathways,” McIntrye said, adding that many people who don’t benefit from current antipsychotic medications display symptoms strongly associated with immune dysregulation.
These treatments could have more powerful effects for people hospitalized with schizophrenia, as well as those who don’t respond to current treatments.
“What’s most debilitating is the cognitive symptoms: that is the ability to focus, concentrate, your memory, your processing speed, and also what’s called negative symptoms,” McIntyre said. “In other words, people who have schizophrenia often have this lack of motivation.”
In 2014, two treatment-resistant patients received an antibody against the protein interferon gamma-1b—responsible for amplifying the body’s immune response. Both patients showed improvements in symptoms over seven weeks.
Two years later, researchers ran an eight-week clinical trial, finding that tocilizumab, a type of antibody-based drug that reduces inflammation approved for treating arthritis, improved cognitive symptoms in schizophrenia patients. The antibody-based drug works by blocking the activity of the interleukin-6 receptor, preventing the activation of the body’s inflammatory response. But a 2018 study involving 36 patients didn’t find any improvement from tocilizumab.
Still, more trials are underway, recruiting more patients to test existing immune-modulating drugs against schizophrenia. One antibody-based drug targeting interleukin-6 and may augment the effects of antipsychotic treatments. Another targets activated microglia, is also being tested in early trials. McIntyre expects that new treatments may be approved by the FDA in the next five or 10 years.
With all that we know so far, it is still difficult to develop better treatments. The immune system is complex and there are many different ways that it can go haywire. The NCAM1 autoantibodies were only present in around 6 percent of people with schizophrenia within the study. Personalized approaches to treating schizophrenia could target the specific aspects of the immune system that aren’t functioning, whether it is autoantibodies, microglia or specific cytokines.
“There are certain forms of breast cancer, you can genotype somebody, and based on the genotype, you can determine whether they are especially going to respond to certain drugs, for example, breast cancer,” McIntyre said. “We’re not there yet in psychiatry, but that’s exactly the direction people want to go.”