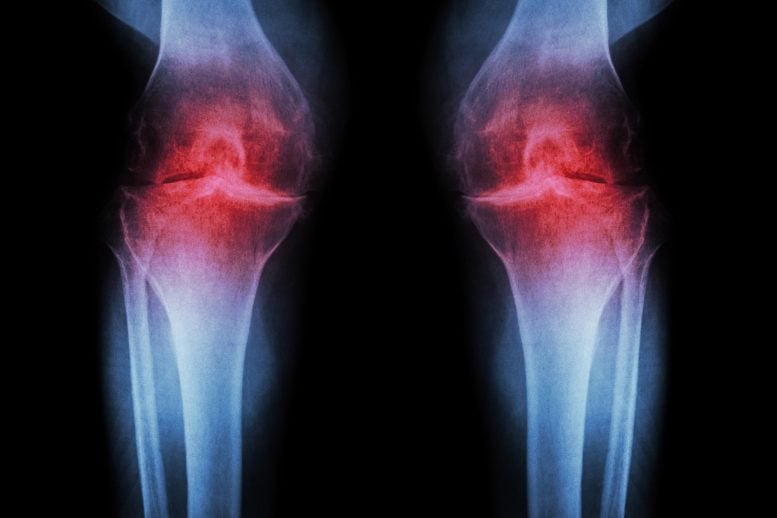
A treatment that blocks an age-related protein restored cartilage in aging and injured joints by reprogramming existing cells rather than using stem cells.
Researchers at Stanford Medicine report that blocking a protein linked to aging can restore cartilage that naturally wears away in the knees of older mice. In the study, the injectable treatment not only rebuilt cartilage but also stopped arthritis from developing after knee injuries similar to ACL tears, which are common among athletes and active adults. A pill-based version of the same therapy is already being tested in clinical trials aimed at treating muscle weakness associated with aging.
Human knee tissue collected during joint replacement surgeries also responded positively to the treatment. These samples, which include both the joint’s supporting extracellular scaffolding, or matrix, and cartilage-producing chondrocyte cells, began forming new cartilage that functioned normally.
Together, these findings point to the possibility that cartilage lost through aging or arthritis could one day be restored using a localized injection or an oral medication, potentially eliminating the need for knee or hip replacement surgery.
Targeting the Root Cause of Osteoarthritis
Rather than easing symptoms, the treatment works by addressing the underlying driver of osteoarthritis. This degenerative joint condition affects roughly one in five adults in the United States and generates an estimated $65 billion in direct health care costs each year. At present, no available medication can halt or reverse the disease, leaving pain management and joint replacement as the primary treatment options.
The therapy targets a protein called 15-PGDH, which becomes more abundant as the body ages and is classified as a gerozyme. Gerozymes, first described by the same research team in 2023, play a central role in aging by contributing to the gradual decline of tissue function. In mice, rising levels of 15-PGDH are a key factor in the loss of muscle strength that occurs with age. When scientists block this protein using a small molecule, older mice show gains in muscle mass and endurance. In contrast, forcing young mice to produce 15-PGDH causes their muscles to weaken and shrink. The protein has also been linked to the regeneration of bone, nerve, and blood cells.
In those tissues, repair depends on the activation and specialization of tissue-specific stem cells. Cartilage behaves differently. Instead of relying on stem cells, chondrocytes alter their gene activity in ways that restore a more youthful state, allowing regeneration to occur without stem cell involvement.
“This is a new way of regenerating adult tissue, and it has significant clinical promise for treating arthritis due to aging or injury,” said Helen Blau, PhD, professor of microbiology and immunology. “We were looking for stem cells, but they are clearly not involved. It’s very exciting.”
Blau, who directs the Baxter Laboratory for Stem Cell Biology and is the Donald E. and Delia B. Baxter Foundation Professor, and Nidhi Bhutani, PhD, associate professor of orthopaedic surgery, are the senior authors of the research, which was in Science. Instructor of orthopaedic surgery Mamta Singla, PhD, and former postdoctoral scholar Yu Xin (Will) Wang, PhD, are the lead authors of the study. Wang is now an assistant professor at the Sanford Burnham Institute in San Diego.
‘Dramatic regeneration’
“Millions of people suffer from joint pain and swelling as they age,” Bhutani said. “It is a huge unmet medical need. Until now, there has been no drug that directly treats the cause of cartilage loss. But this gerozyme inhibitor causes a dramatic regeneration of cartilage beyond that reported in response to any other drug or intervention.”
There are three main types of cartilage in the human body. One, elastic cartilage, is soft and flexible and forms structures like the outer ear. A second, fibrocartilage, is dense and tough, absorbing shock in areas such as between the spinal vertebrae. The third, hyaline cartilage, is smooth and glossy, providing a low-friction surface for lubrication and flexibility in joints like the ankles, hips, shoulders and parts of the knee. Hyaline cartilage — also known as articular cartilage — is the cartilage most commonly affected by osteoarthritis.
Osteoarthritis occurs when a joint is stressed by aging, injury or obesity. The chondrocytes begin to release pro-inflammatory molecules and to break down collagen, which is the primary structural protein of cartilage. When collagen is lost, the cartilage thins and softens; the accompanying inflammation causes the joint swelling and pain that are hallmarks of the disease. Under normal circumstances, articular cartilage rarely regenerates. Although some populations of putative stem or progenitor cells capable of generating cartilage have been identified in bone, attempts to identify similar populations of cells in the articular cartilage have been unsuccessful.
Previous research from Blau’s lab has shown that a molecule called prostaglandin E2 is essential to muscle stem cell function. 15-PGDH degrades prostaglandin E2. Inhibiting 15-PGDH activity, or increasing levels of prostaglandin E2, supports the regeneration of damaged muscle, nerve, bone, colon, liver and blood cells in young mice.
Blau, Bhutani and their colleagues wondered if 15-PGDH might also play a role in aging cartilage and joints. They wanted to find out if a similar pathway contributes to cartilage loss from aging or in response to injury. When they compared the amount of 15-PGDH in the knee cartilage in young versus old mice, they saw that, as in other tissues, levels of the gerozyme increased about two-fold with age.
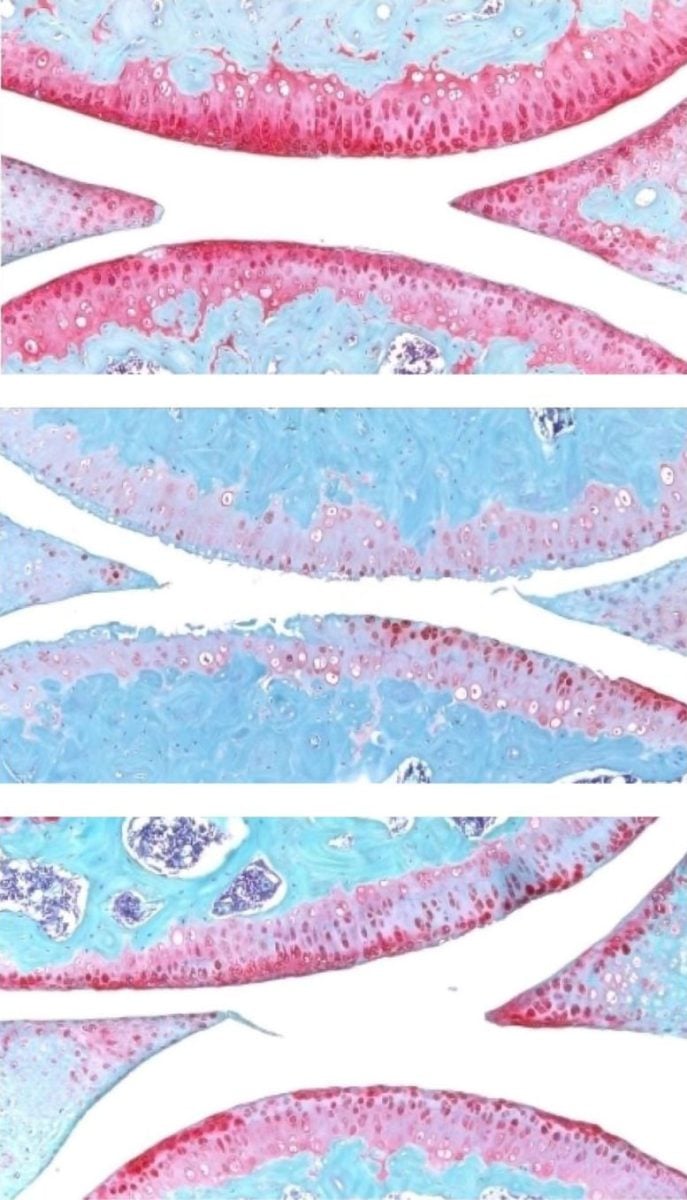
They next experimented with injecting old animals with a small molecule drug that inhibits 15-PGDH activity — first into the abdomen, which affects the entire body, then directly into the joint. In each case, the knee cartilage, which was markedly thinner and less functional in older animals as compared with younger mice, thickened across the joint surface. Further experiments confirmed that the chondrocytes in the joint were generating hyaline, or articular, cartilage, rather than less-functional fibrocartilage.
“Cartilage regeneration to such an extent in aged mice took us by surprise,” Bhutani said. “The effect was remarkable.”
Addressing ACL tears
Similar results were observed in animals with knee injuries like the ACL tears that frequently occur in people participating in sports such as soccer, basketball and skiing that require sudden pivoting, stopping or jumping. While the tears can be surgically repaired, about 50% of people develop osteoarthritis in the injured joint within about 15 years.
The researchers found that a series of injections twice a week for four weeks of the gerozyme inhibitor after injury dramatically reduced the chance that osteoarthritis develops in the mice. Animals treated with a control drug had levels of 15-PGDH that were twice as high as in their uninjured peers, and they developed osteoarthritis within four weeks.
The animals treated with the gerozyme inhibitor also moved more typically and put more weight on the paw of the affected leg than did untreated animals.
“Interestingly, prostaglandin E2 has been implicated in inflammation and pain,” Blau said. “But this research shows that, at normal biological levels, small increases in prostaglandin E2 can promote regeneration.”
A closer investigation of the chondrocytes in the joints of old mice and young mice showed that old chondrocytes expressed more detrimental genes involved in inflammation and the conversion of hyaline cartilage to unwanted bone, and fewer genes involved in cartilage development.
Reprogramming Cartilage Cells Without Stem Cells
The researchers were also able to pinpoint subcategories of old chondrocytes that change their patterns of gene expression after treatment. One, which expresses 15-PGDH and genes involved in cartilage degradation, decreased in prevalence from 8% to 3% after treatment. Another, which does not express 15-PGDH but does express genes involved in the production of fibrocartilage, also decreased in prevalence: from 16% to 8% after treatment.
A third population, which does not make 15-PGDH and which expresses genes involved in hyaline cartilage formation and the maintenance of the extracellular matrix necessary for its function, increased in prevalence after treatment from 22% to 42%. The findings indicate an overall shift in gene expression after treatment to a more youthful cartilage composition — without the involvement of stem or progenitor cells.
Finally, the researchers studied human cartilage tissue removed from patients with osteoarthritis undergoing total knee replacements. Tissue treated with the 15-PGDH inhibitor for one week exhibited lower levels of 15-PGDH-expressing chondrocytes and lowered cartilage degradation and fibrocartilage genes than control tissue and began to regenerate articular cartilage.
“The mechanism is quite striking and really shifted our perspective about how tissue regeneration can occur,” Bhutani said. “It’s clear that a large pool of already existing cells in cartilage are changing their gene expression patterns. And by targeting these cells for regeneration, we may have an opportunity to have a bigger overall impact clinically.”
Blau added, “Phase 1 clinical trials of a 15-PGDH inhibitor for muscle weakness have shown that it is safe and active in healthy volunteers. Our hope is that a similar trial will be launched soon to test its effect in cartilage regeneration. We are very excited about this potential breakthrough. Imagine regrowing existing cartilage and avoiding joint replacement.”
Reference: “Inhibition of 15-hydroxy prostaglandin dehydrogenase promotes cartilage regeneration” by Mamta Singla, Yu Xin Wang, Elena Monti, Yudhishtar Bedi, Pranay Agarwal, Shiqi Su, Sara Ancel, Maiko Hermsmeier, Nitya Devisetti, Akshay Pandey, Mohsen Afshar Bakooshli, Adelaida R. Palla, Stuart Goodman, Helen M Blau and Nidhi Bhutani, 27 November 2025, Science.
DOI: 10.1126/science.adx6649
Researchers from the Sanford Burnham Prebys Medical Discovery Institute contributed to the work.
The study was funded by the National Institutes of Health (grants R01AR070864, R01AR077530, R01AG069858 and R00NS120278), the Baxter Foundation for Stem Cell Biology, the Li Ka Shing Foundation, the Stanford Cardiovascular Institute, the Milky Way Research Foundation, the Canadian Institutes of Health Research, a Stanford Translational Research and Applied Medicine Pilot grant, a GlaxoSmithKline Sir James Black Postdoctoral Fellowship, and a Stanford Dean’s Postdoctoral Fellowship.
Blau, Bhutani and other co-authors are inventors on patent applications held by Stanford University regarding 15-PGDH inhibition in cartilage and the rejuvenation of tissues and organs licensed to Epirium Bio. Blau is a co-founder of Myoforte/Epirium, and she holds equity and stock options in the company.
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google and Google News.